After learning about diffusion and osmosis, today inclass we went onto the topic of rate of diffusion, as explained below:
Rate of Diffusion
Since the average kinectic enerygy of different types of molecules (different masses) which are at thermal equilibrium is the same, then their average velocities are different. Their average diffusion rate is expected to depend upon that average velocity, which gives a relative diffusion rate
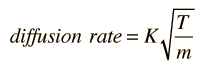
where the constant K depends upon geometric factors including the area across which the diffusion is occuring. The relative diffusion rate for two different molecular species is then given by
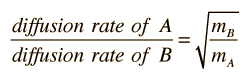 Reference : http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/diffus.html
Reference : http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/diffus.html
We did a lab that was done the first time ever at Webb Schools of california, it was a lab to check the rate of diffusion of something and determine what is the best shape for a cell to acquire maximum performance on rate of diffusion.
We were given three cubes of 3% agar-phenolphthalein in different sizes and also some NaOH solute, since phenolphthalein reacts with NaOH and when it does it turns pink, we can determine how fast did the rate of diffusion happened by using three cubes of different sizes and lengths to determine the best shape.
So first, we put the blocks into the NaOH solution,
Then after 3 minutes we took the cubes out of the beakers and cut them in halves then measured them:
After the measurements, we realized that the smaller the cube is the faster the rate of diffusion and thus explaining why cell division happens. Because cells needs their nucleus to be in a reaction in order to react therefore having a smaller volume allows other chemicals to penetrate in the cell easier.
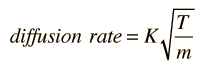
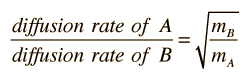


The rate of diffusion is not different but the smaller area for diffusion to occur the greater the chance that the area will have the substance diffuse fully in.
ReplyDelete